How to Receive the Economic Compensation your Exactech Ankle Implant Case Deserves

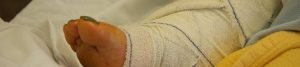
An Exactech, Inc. recall announcement states that the implant manufacturer is recalling over 140,000 ankle and knee implant systems due to defective Exactech polyethylene (plastic) inserts. In addition, Exactech discovered that the ankle implants, including Exactech vantage, were packaged in packaging that caused some of the implants and polyethylene (plastic) inserts to be exposed to oxygen leading to rapid degradation and some patients may be required to undergo revision surgery. In some cases, implant patients may also suffer some bone loss, requiring additional surgery. You can contact us to learn more about the Exactech ankle replacement lawsuit.
The Exactech implant recall announcement states that the following ankle and knee implants manufactured since 2004 are being recalled:
If you or a family member have received one of these recalled ankle or knee implants, there is a chance that your implant is faulty and needs to be replaced.
What is the Problem With These Exactech Ankle and Knee Implants?
According to Exactech’s recall announcement, the recalled implants had been packaged in “non-conforming” packaging, and this packaging failed to protect the implants from oxygen. When the implants are exposed to oxygen before implantation, they might begin to oxidize. When these implants begin to oxidize, the implants could start breaking down, accelerate the production of wear debris, crack, and/or cause bone loss. The improper packaging also did not contain a second barrier with ethylene-vinyl alcohol, which may have prevented oxygen exposure prior to “partial” and “total” knee and ankle revision/replacement surgeries.
The recall announcement also states that the polyethylene (plastic) inserts that fit between the Talar component and the Tibial component within the ankle joint might wear out prematurely when exposed to oxygen before implantation. Patients who are affected by the Exactech ankle recall may receive a recall letter from the Exactech explaining the issue and what ankle implant patients should do, especially if they are feeling symptoms of a failing ankle implant. Ankle implant patients who are suffering from the following symptoms should immediately seek a medical assessment and treatment. The signs and symptoms of ankle implant premature failure and/or wear might include:
- Redness,
- swelling,
- unusual and ongoing pain,
- ankle joint instability, and/or stiffness.
Ankle implant patients who are not suffering from the symptoms mentioned above should still speak with their surgeon about this ankle implant recall at the next scheduled implant checkup. The recall announcement and letters state that it is important that patients also receive an x-ray examination of their ankle implant, even if they do not suffer any symptoms of a worn-out or failing implant.
How to Recover Monetary Damages from the Exactech Ankle Replacement Lawsuit
Ankle implant patients and their surgeons place trust in implant makers to manufacture safe, effective surgical implants. Implant patients suffer tremendously when an implant manufacturer fails to ensure that their products are safe and effective. If you received one of the recalled Exactech Vantage ankle implants, it is crucial to speak with your surgeon to see if your implant is part of this ankle implant recall. If your ankle implant is being recalled, your surgeon should examine your implant and determine if or when you need revision surgery.
Patients who are implanted with a recalled Exactech Vantage ankle implant should discuss their claim with our product liability attorneys to decide if they qualify to file an Exactech Vantage ankle implant lawsuit to pursue justice and monetary compensation. Surgical implant makers could be held responsible for the harm their defective products cause others. Our product liability attorneys are here to help you or your family member understand the legal process.
Why Choose Parker Waichman LLP?
At Parker Waichman LLP, our Exactech Vantage Total Ankle Implant Lawsuit Lawyers have substantial experience representing clients in product liability cases, including lawsuits involving recalled or defective implants. You can be assured that our attorneys will fight hard to achieve the most favorable possible results in your ankle implant case. An ankle implant failure must not be taken lightly. Take action today and pursue the compensation that your case deserves by calling our firm.
At Parker Waichman LLP, our product liability law firm is dedicated to achieving the best results for each and every client. Our law firm has recovered over $2 billion for our clients. Parker Waichman LLP has been awarded several honors and accolades, such as:
- An AVVO rating of 9.8 out of 10.
- A listing in Best Lawyers, which is based on extensive peer review.
- A peer review rating of “AV Preeminent,” which is the highest rating, from Martindale-Hubbell.
- Law dragon’s highest ranking of “5 Dragons.”
If a defective Exactech Vantage Ankle Revision Implant has harmed you or a member of your family, choose Parker Waichman LLP. Our Exactech lawsuit attorneys will listen, conduct an investigation, and work hard to obtain results.
Get Help with Your Exactech Ankle Replacement Lawsuit
If you or a family member sustained an injury or needed revision surgery due to a defective Exactech Vantage Ankle Implant, contact Parker Waichman LLP for a free consultation. Our legal professionals are here to help. Contact our defective medical devices attorney for your free consultation by chatting with our online chat, filling out our online contact form, or by calling 800-968-7529.